We just closed the books on the first quarter of 2019 and our key products are helping to deliver another strong start for Abbott.
For the quarter, we delivered sales growth of more than 7% (on an organic basis) and exceeded our previous ongoing earnings-per-share guidance thanks to key products such as FreeStyle Libre, Alinity and MitraClip. We are also seeing several other bright spots throughout our portfolio in our medical devices, nutrition and established pharma businesses.
And, our innovative pipeline and focused execution is continuing to pay off. We saw more than 10 significant product approvals and developments in the first quarter alone.
Some of the highlights include:
For full financials, you can read our press release, or take a look at some additional materials below:
Abbott's Chairman and CEO Miles D. White shared his views on first-quarter performance:

Learn more about Abbott's first-quarter 2019 results:
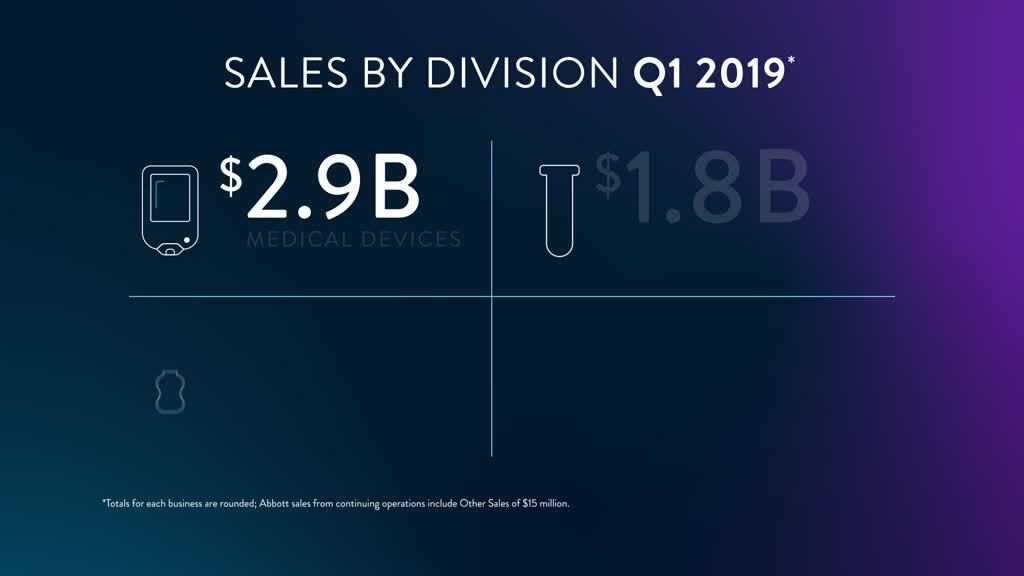
Download a summary of Abbott's earnings highlights here.
For more information on Abbott's earnings results, see the news release.
*Alinity m is in development and not commercially available in the United States for diagnostic use.
Please be aware that the website you have requested is intended for the residents of a particular country or region, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions.
The website you have requested also may not be optimized for your specific screen size.
Share